Research / Clinical Trials

Brenda Whitehead, CCRP, Kimp Puccio, LPN CRC and Lisney Dunlap, CMA CRC
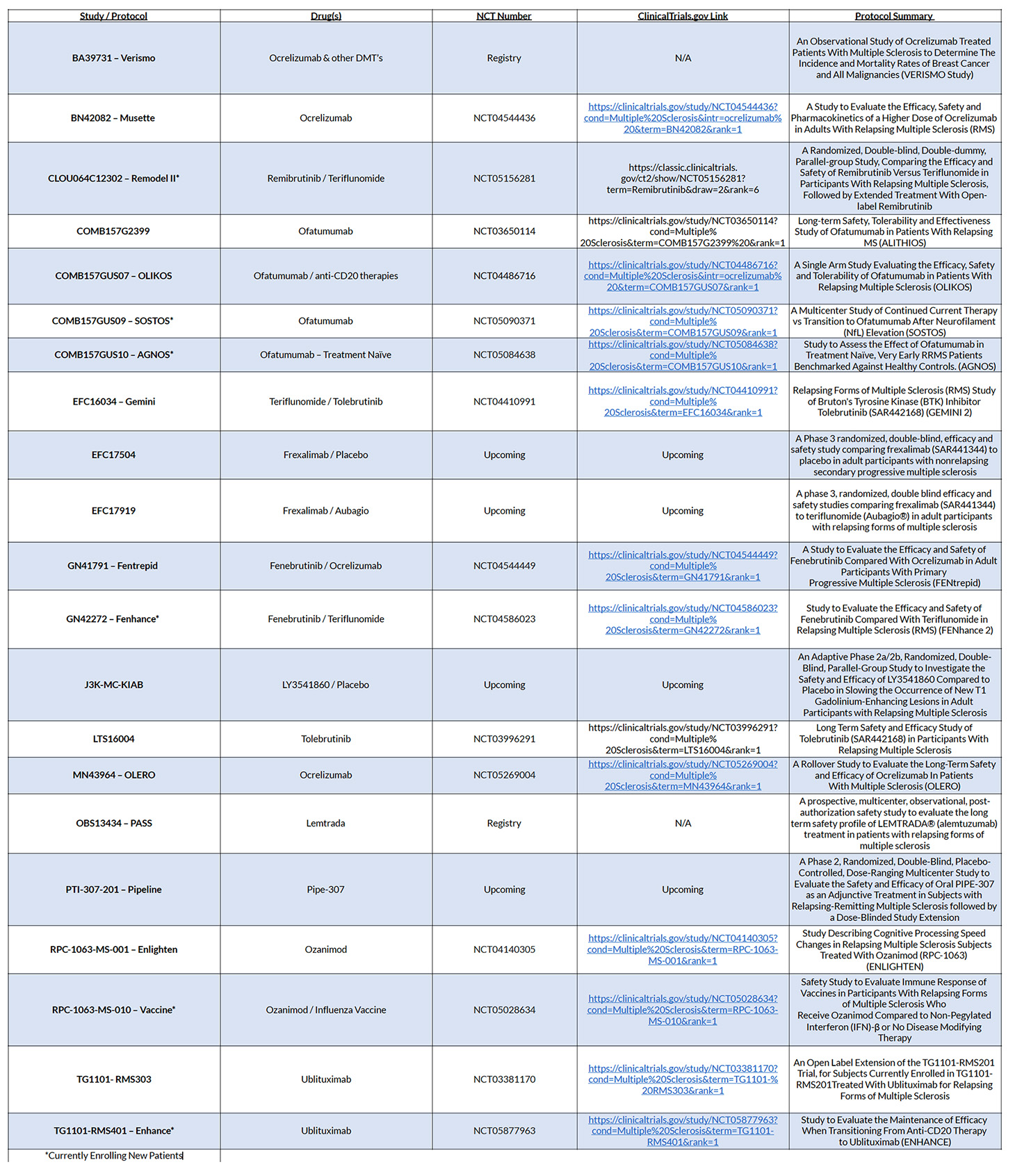
Our practice offers clinical trials which help determine whether newly developed treatments are safe and effective in treating Multiple Sclerosis. These studies are important research tools for developing new treatment options for this chronic disease. Clinical trials also provide patients with access to new treatment options and new therapies that are not offered to the general population.
As new technologies and therapeutic options become available, Hope Neurology Multiple Sclerosis Center utilizes clinical trials to provide innovative treatment options to our patients.
Clinical research studies provide patients with new medications and access to comprehensive disease management. By working closely with our pharmaceutical sponsors, we strive to improve the health, hope and well-being of our patients.
For more information on any of our studies, please call 865-218-6222 and ask to speak with Kim Puccio, Sydney Brawner, or Jada Thomas or email us at hnresearch@hopeneuro.com .
CONTACT INFORMATION
CLINICAL TRIALS | UPDATED TRIAL LIST – 05 July 2023